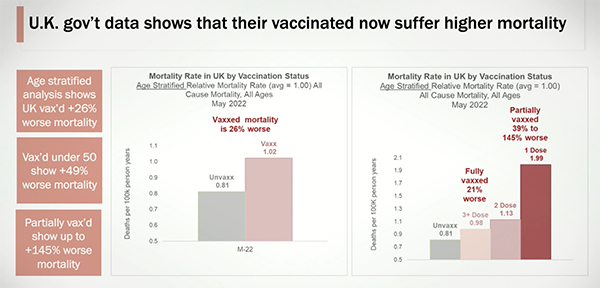
Report: FDA’s failure to adequately oversee vaccine clinical trials is endangering public health
11/28/2022 / By Olivia Cook

A paper has revealed that the Food and Drug Administration (FDA) exhibited a lax attitude toward safety concerns about the Wuhan coronavirus (COVID-19) vaccines, which the agency later approved.
The said paper by Australian investigative journalist Maryanne Demasi was published Nov. 16 in BMJ Investigation. She revealed that the FDA exhibited a lax attitude regarding the oversight of COVID-19 vaccine and drug trials.
“Regulatory documents show that only nine out of 153 Pfizer trial sites were subject to FDA inspection before licensing the mRNA vaccine. Similarly, only 10 out of 99 Moderna trial sites and five of 73 remdesivir trial sites were inspected,” Demasi wrote.
The journalist cited a September 2020 complaint lodged to the FDA by Brook Jackson, a regional director working for the Texas-based Ventavia Research Group. Ventavia had been hired to run clinical trials for the Pfizer BNT162b2 mRNA vaccine.
Jackson witnessed problems at three trial sites she was overseeing – such as falsified data, unblinded patients, and inadequately trained vaccinators who were slow to follow up on adverse events. She brought this to the attention of an FDA inspector, but the regulator did not inspect the trial site in question.
“I thought that the FDA was going to swoop in and take care of everything,” Jackson said. “What I was reporting was so important.”
A former staffer in the FDA’s Office of Criminal Investigations raised red flags over the agency’s failure to act on Jackson’s complaints.
“Having worked at the FDA, I see it as surprising, for many reasons, that the agency turned a blind eye. They likely feared the criticism they undoubtedly would have received for holding up a vaccine, which they knew they would eventually approve anyway, at the expense of untold lives lost,” the anonymous staffer said.
“My point here is that instead of the regulators protecting the public, they were complicit. At the time, they may have been doing what they believed to be the right thing under extraordinary circumstances. But now, they may soon have some explaining to do. (Related: Pfizer, FDA know the COVID vaccine is dangerous, but they push it away.)
Former FDA advisor accuses agency of “endangering public health”
David Gortler, a former senior advisor to the FDA commissioner, remarked that the regulator is “endangering public health” by not being candid about the violations uncovered during clinical trial site inspections. He was appointed to the advisory position from 2019 until 2021, and was previously an FDA medical reviewer from 2007 to 2011.
“The lack of full transparency and data sharing does not allow physicians and other medical scientists to confirm the data independently and make comprehensive risk-benefit assessments,” added Gortler, now a fellow at the Washington, D.C.-based think tank Ethics and Public Policy Center (EPPC).
The FDA paused site inspections during the peak of COVID-19 pandemic restrictions between March and July 2020, with only “mission-critical inspections” being carried out. However, Gortler said the regulator should have ramped up its oversight instead of scaling back. He added that COVID-19 products intended for millions of people were being developed at “warp speed,” and the FDA ought to catch up.
“The drug companies took appropriate measures to keep staff safe, which is exactly what the FDA could and should have done,” Gortler said.
Speaking to the BMJ, the FDA reiterated that it takes oversight of clinical trials seriously. It also mentioned publishing draft guidance for remote regulatory assessments – including virtual inspections using live streaming and video conferencing, and requests to view records remotely.
Gortler, a credentialed FDA inspector, laughed at the proposition.
“You can’t do a remote inspection. That’s like saying ‘I’m going to arrest somebody remotely.’ You have to be there on site and look at every nuance such as cleanliness, organization, staff coordination – even their body language,” he said.
“During a pandemic, the FDA could’ve put inspectors in hazmat suits if they wanted to. There’s no excuse for not going onsite.”
Watch Brook Jackson reveal the extent of Pfizer’s COVID-19 vaccine fraud to Australian independent journalist Maria Zeee below.
This video is from the KristallKlar channel on Brighteon.com.
More related stories:
Smoking gun? FDA refusing to provide key COVID “vaccine” safety analysis suggesting massive coverup.
Walensky admits CDC spread MISINFORMATION on COVID vaccine safety.
More COVID vaccine negative effects and coverups are emerging.
Sources include:
Submit a correction >>
Tagged Under:
big government, Big Pharma, Brook Jackson, clinical trial, conspiracy, covid-19, FDA, Maryanne Demasi, oversight, pandemic, Pfizer, rigged, science deception, science fraud, site inspections, vaccine, vaccine safety, Whistleblower, Wuhan coronavirus
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
PharmaceuticalFraud.com is a fact-based public education website published by Pharmaceutical Fraud Features, LLC.
All content copyright © 2018 by Pharmaceutical Fraud Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.