Over 2M at-home COVID-19 test kits recalled due to false positive results
11/17/2021 / By Mary Villareal
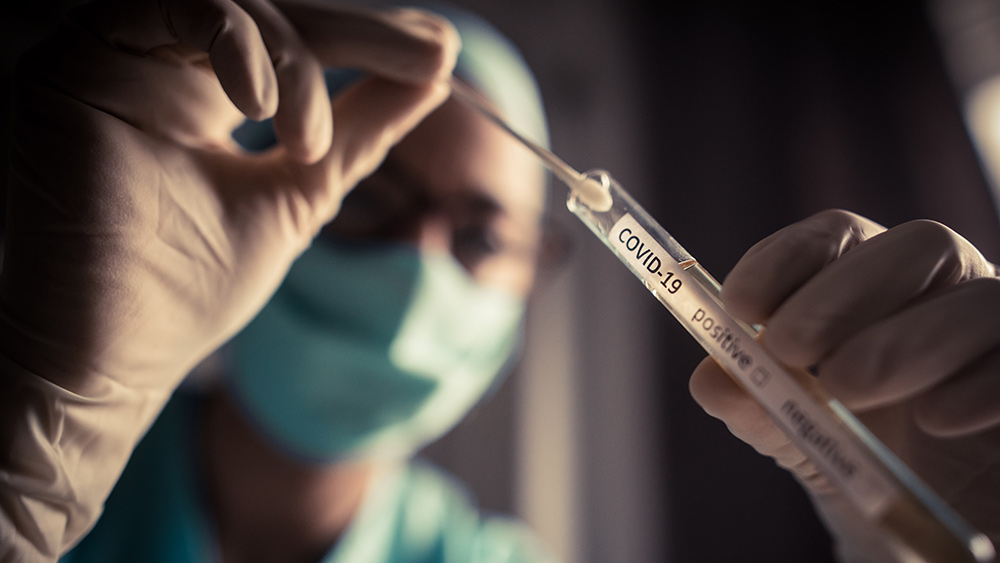
The Food and Drug Administration (FDA) recalled some 2.2 million Ellume at-home COVID-19 test kits due to a manufacturing defect that causes false positive results. The firm first informed the regulatory agency about the defect in October.
Ellume is the first company to get FDA approval for over-the-counter COVID tests. The defective test kits were manufactured between February 24 and August 11. The recall of the 2.2 million test kits is an expansion of last month’s recall of 200,000 kits for the same reason.
False positive results indicate that a person has contracted the virus that causes COVID-19 when they actually have not. The FDA noted that these false positives could lead to delayed diagnosis or treatment for the actual cause of the person’s illness, which could be a different life-threatening disease other than COVID-19. They may also receive unnecessary COVID-19 treatment from a health care provider, which may result in adverse effects.
Another problem that the FDA pointed out is the isolation, including monitoring household members or close contacts for symptoms, limiting contact with family and friends and missing work or school.
Due to the severity of the issue, the FDA said it is being identified as a Class I recall, or the most serious type, as these tests may cause serious adverse health consequences or death. The antigen test detects proteins from the virus from a nasal sample and is available without prescription for use by individuals aged two and older. The kit comes with an analyzer that connects to a smartphone app to show users how to perform the test and understand the results.
The Biden administration signed a $231 million deal with the company, which received approval to produce its test under the Trump administration last year. In October, Ellume Chief Executive, Dr. Sean Parsons said that the company created more safeguards to ensure that the problem does not happen again.
“I’m very sorry that this has happened. We’re all about chasing accuracy, and to have these false positives is disappointing,” he said. (Related: Australian COVID-19 test kit manufacturer recalls 195,000 at-home kits due to high rate of false positives.)
A spokesperson added that the “root cause” of the issue has been identified and that the company is already on the way to shipping new products to the United States.
Consumers encouraged to contact health care providers
Consumers who received positive results within the last two weeks using the Ellume test kits are encouraged to contact their health care providers despite the fact that the “incidence of false positives is limited to specific lots,” according to Parsons.
“The FDA is continuing to work with Ellume to assess the company’s corrective actions, such as additional manufacturing checks and other corrective steps, to address the reason for the manufacturing issue, and to help ensure that it is resolved and will not recur,” the agency said.
The recall comes at a time when it is not easy to find at-home tests in the country. Many pharmacies are out of stock or have expensive brands available – some costing about $24 per pair, which is an expensive outlay for those who need frequent testing.
While many would like to test for COVID-19 at home, the shortages and costs mean that many check their health status only when traveling or attending special events. However, this could change in the next few months as the Biden administration announced its $1 billion investment to expand the supply of such test kits.
Get more news related to COVID-19 at Pandemic.news.
Sources include:
Tagged Under: at-home test, coronavirus, coronavirus tests, covid-19, covid-19 testing, Ellume, false-positive, FDA, infections, products, science deception, science fraud
RECENT NEWS & ARTICLES
PharmaceuticalFraud.com is a fact-based public education website published by Pharmaceutical Fraud Features, LLC.
All content copyright © 2018 by Pharmaceutical Fraud Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.